Answer:
The age of living tree is 11104 years.
Step-by-step explanation:
Given that,
Mass of pure carbon = 100 g
Activity of this carbon is = 6.5 decays per second = 6.5 x60 decays/min =390 decays/m
We need to calculate the decay rate
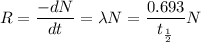 ....(I)
....(I)
Where, N = number of radio active atoms
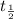 =half life
=half life
We need to calculate the number of radio active atoms
For
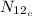
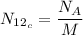
Where,
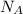 =Avogadro number
=Avogadro number
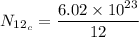
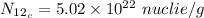
For
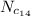
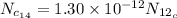
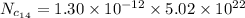
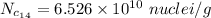
Put the value in the equation (I)
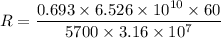
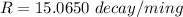
100 g carbon will decay with rate
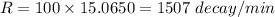
We need to calculate the total half lives
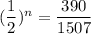
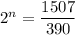
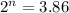
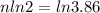
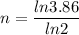
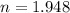
We need to calculate the age of living tree
Using formula of age
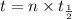
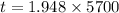
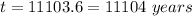
Hence, The age of living tree is 11104 years.