Answer: Option (A) is the correct answer.
Step-by-step explanation:
According to Le Chatelier's principle, any disturbance in an equilibrium reaction will shift the equilibrium in the direction that will be opposing the change.
For example, in the reaction
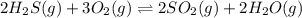
As steam means that water is present in gaseous form. So, here steam is present on the product side that is, right hand side. Hence, increasing concentration of steam will shift the equilibrium on left hand side, that is, on the reactant side.
Thus, we can conclude that in the given reaction adding steam to the reaction vessel will shift the reaction to the left.