Answer:
option d is correct
work done is 647 J
Step-by-step explanation:
Given data
pressure P = 3 atm
volume V1 = 2 L
volume V2 = 8 L
to find out
work is done by the gas
solution
we know that work done formula that is
work done = k (
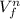 -
-
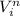 ) / n
) / n
here n = 1 - γ
and k = PV^γ
and we know for diatomic gas γ = 1.4
so k will be = ( 3× 101325 ) ×
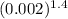
k = 50.62
so that now
work done = k (
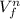 -
-
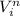 ) / n
) / n
work done = 50.62 (
 -
-
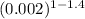 ) / 1-1.4
) / 1-1.4
so work done will be 647 J
so option d is correct
work done is 647 J