Answer:
Both reaction A and reaction B are non spontaneous.
Step-by-step explanation:
For a spontaneous reaction, change in gibbs free energy (
 ) should be negative.
) should be negative.
We know,
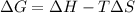 , where T is temperature in Kelvin scale.
, where T is temperature in Kelvin scale.
Reaction A:
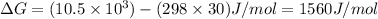
As
 is positive therefore the reaction is non-spontaneous.
is positive therefore the reaction is non-spontaneous.
If at a temperature T K , the reaction is spontaneous then-
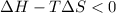
or,
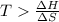
or,
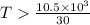
or,
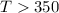
So at a temperature greater than 350 K, the reaction is spontaneous.
Reaction B:
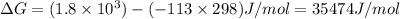
As
 is positive therefore the reaction is non-spontaneous.
is positive therefore the reaction is non-spontaneous.
If at a temperature T K , the reaction is spontaneous then-
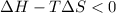
or,
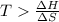
or,
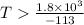
or,
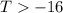
So at a temperature greater than -16 K, the reaction is spontaneous.