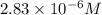Step-by-step explanation:
a) Using Beer-Lambert's law :
Formula used :
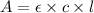
where,
A = absorbance of solution = 0.945
c = concentration of solution = ?
l = length of the cell = 1.20 cm
 = molar absorptivity of this solution =
= molar absorptivity of this solution =
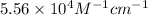
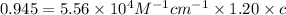
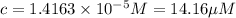
(
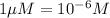 )
)
14.16 μM is the molarity of the red dye solution at the optimal wavelength 519nm and absorbance value 0.945.
b)
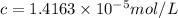
1 L of solution contains
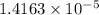 moles of red dye.
moles of red dye.
Mass of
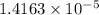 moles of red dye:
moles of red dye:
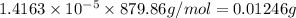
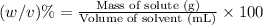
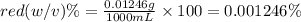
c) In order to dilute red dye solution by 5 times, we will need to add 1 L of water to solution of given concentration.
Concentration of red dye solution =
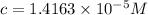
Concentration of red solution after dilution = c'
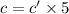
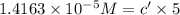
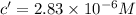
The final concentration of the diluted solution is