Step-by-step explanation:
We assume that
 is represented by A and
is represented by A and
 is represented by B respectively.
is represented by B respectively.
According to Wilke Chang equation as follows.
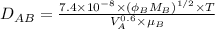
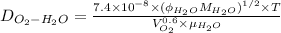
where, T = absolute temperature = (273 + 37)K = 310 K
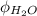 = an association parameter for solvent water = 2.26
= an association parameter for solvent water = 2.26
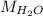 = Molecular weight of water = 18 g/mol
= Molecular weight of water = 18 g/mol
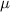 = viscosity of water (in centipoise) = 0.62 centipoise
= viscosity of water (in centipoise) = 0.62 centipoise
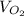 = the molar volume of oxygen = 25.6
= the molar volume of oxygen = 25.6
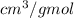
Hence, putting the given values into the above formula as follows.
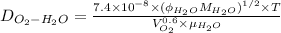
=
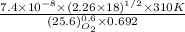
=
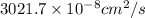
Thus, we can conclude that the diffusion of
 in water by the Wilke-Chang correlation at
in water by the Wilke-Chang correlation at
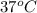 .
.