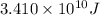Answer : The energy produced is
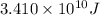
Explanation :
First we have to calculate the moles of
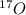 .
.
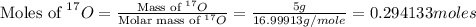
Now we have to calculate the mass defect.
The balanced reaction is,
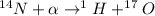
Mass defect = Sum of mass of product - sum of mass of reactants
![\Delta m=[(n_(^1H)* M_(^1H))+(n_(^(17)O)* M_(^(17)O))]-[(n_(^(14)N)* M_(^(14)N))+(n_(\alpha)* M_(\alpha))]](https://img.qammunity.org/2020/formulas/chemistry/college/j4eu83j2wreqzy9qg9n8o0g9xw2wgth1of.png)
where,
n = number of moles = 0.294133 moles
M = molar mass
Now put all the given values in the above, we get:
![\Delta m=[(n_(^1H)* M_(^1H))+(n_(^(17)O)* M_(^(17)O))]-[(n_(^(14)N)* M_(^(14)N))+(n_(\alpha)* M_(\alpha))]](https://img.qammunity.org/2020/formulas/chemistry/college/j4eu83j2wreqzy9qg9n8o0g9xw2wgth1of.png)
![\Delta m=[(0.294133mole* 1.00783g/mole)+(0.294133mole* 16.99913g/mole)]-[(0.294133mole* 14.00307g/mole)+(0.294133mole* 4.0026g/mole)]](https://img.qammunity.org/2020/formulas/chemistry/college/7aol74skgiokb1wopi2edbc96h82aytqk1.png)
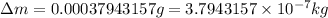
Now we have to calculate the energy produced.
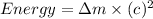
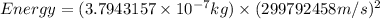
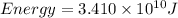
Therefore, the energy produced is