Step-by-step explanation:
The given data is as follows.
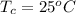 ,
,
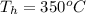
Work produced per kJ of heat extracted from hot reservoir = 0.45 kJ = Efficiency
If the device is Carnot cycle then its efficiency will be maximum and its value will be equal to
![[1 - ((T_(c))/(T_(h)) )]](https://img.qammunity.org/2020/formulas/chemistry/college/2kp9ccj6arx60r9mpet02bl4jrnnwldt8c.png)
Using this relation we will calculate the efficiency as follows.
Efficiency =
![[1 - ((T_(c))/(T_(h)) )]](https://img.qammunity.org/2020/formulas/chemistry/college/2kp9ccj6arx60r9mpet02bl4jrnnwldt8c.png)
=
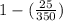
= 0.928
Hence, it means that this type of device is possible and the claim is also believable.