Step-by-step explanation:
1) A balanced equation for the reaction.
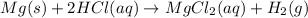
2) The name of the soluble slat produced by reaction between magnesium and hydrochloric acid is magnesium chloride.
Magnesium chloride =
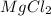
3)Yes, chemical change has been taking.
This is because rearrangement of atom is taking place which resulted in formation of hydrogen gas amd magnesium chloride.
4) Moles of hydrogen gas produced = 2 mole
According to reaction, 1 mole of hydrogen gas is obtained from 2 moles of HCl.
Then 2 moles of hydrogen gas will be obtained form:
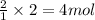 of HCl
of HCl
4 moles of acid has reacted.