Answer:
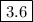
Step-by-step explanation:
2NO₂ ⇌ N₂O₄
E/mol·L⁻¹: 0.058 0.012
K_{\text{eq}} = \dfrac{\text{[N$_{2}$O$_{4}$]}}{\text{[NO$_{2}$]$^{2}$}} = \dfrac{0.012}{0.058^{2}} = \mathbf{3.6} \\\\
\text{The $K_{\text{eq}}$ value would be $\boxed{\mathbf{3.6}}$}