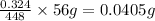Answer:
Amount of elemental iron in sample A is 0.0405g.
Step-by-step explanation:
Molar mass of C = 12 g/mol
Molar mass of H = 1 g/mol
Molar mass of Fe = 56 g/mol
Molar mass of O = 16 g/mol
So, molar mass of ferrous gluconate =
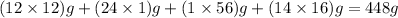
So number of mole of ferrous gluconate in 324 mg =
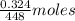
(number of moles =
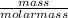 )
)
As 1 mol of ferrous gluconate contains 1 mol of Fe therefore
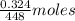 of ferrous gluconate contain
of ferrous gluconate contain
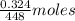 of Fe.
of Fe.
So amount of elemental iron in sample A =