Answer: 4.6 grams
Step-by-step explanation:
1 electron carry charge=
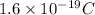
1 mole of electrons contain=
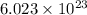 electrons
electrons
Thus 1 mole of electrons carry charge=
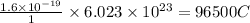
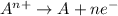
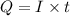
where Q= quantity of electricity in coloumbs
I = current in amperes = 0.800A
t= time in seconds = 100 min= 6000 sec (1min=60sec)
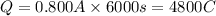
96500C charge is carried by = 1 mole of electrons
4800C charge is carried by=
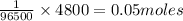
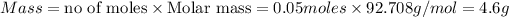
Thus 4.6g of
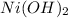 is oxidized to
is oxidized to
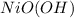 .
.