Answer:
29.38 seconds
Step-by-step explanation:
Half life, T = 22.07 s
No = 1293
Let N be the number of atoms left after time t
N = 1293 - 779 = 514
By the use of law of radioactivity
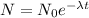
Where, λ is the decay constant
λ = 0.6931 / T = 0.6931 / 22.07 = 0.0314 decay per second
so,
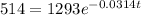
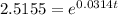
take natural log on both the sides
0.9225 = 0.0314 t
t = 29.38 seconds