Answer: Mg is the excess reactant for the forward reaction.
Explanation: It is a stoichiometry problem and solved with the help of given grams and using balanced equation. Grams of both the reactants are converted to moles and divided by their coefficients. The excess reactant is the one for which we get the highest number on doing above steps.
The balanced equation is:
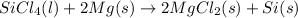
Molar mass of silicon tetra chloride is 169.9 gram per mol and the molar mass of Mg is 24.3 gram per mol.
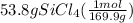
=
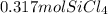
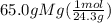
= 2.67 mol Mg
From balanced equation, the coefficient of silicon tetra chloride is 1 and that of Mg is 2. So, we will divide the moles of silicon tetra chloride by 1 and that of Mg by 2 and see which one gives highest number.
For silicon tetra chloride,
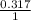 = 0.317
= 0.317
and for Mg,
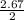 = 1.34
= 1.34
The highest number is for Mg and so the excess reactant for the forward reaction is Mg.