Step-by-step explanation:
The given data is as follows.
Current =
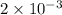 A
A
Time = 10800 sec
Weight of cathode solution = 51.7436 g
Weight of HCl after analysis = 0.0267 g (in cathode solution)
Weight of anode solution = 52.0461 g
Weight of HCl in anode solution = 0.0133 g
Amount of electrolyte present = molality × molar mass
=
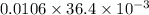
=
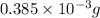
Calculate the amount of HCl initially present as follows.
Electricity passed =
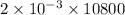 = 21.6 C
= 21.6 C
Hence, amount of electrons passed will be as follows.
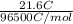
= 0.00024 mol
Therefore, mass of HCl initially present is as follows.
= (51.7436 - 0.0267)
= 51.7169 g of water
=
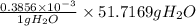
= 0.01994 g
Mass of HCl present after the electrolysis in 51.7169 g of
 = 0.0267 g
= 0.0267 g
Mass of HCl gained = 0.0267 - 0.01994
= 0.00676 g
Hence, the amount of HCl gained =
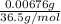
= 0.0001853 mol
Therefore, gain of
 in cathodic compartment = 0.000224 mol
in cathodic compartment = 0.000224 mol
So, transport number of
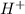 =
=
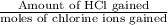
=
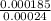
= 0.823
Thus, we can conclude that the transport number of
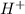 is 0.823.
is 0.823.