Answer:
(a) True
Step-by-step explanation:
The compressibility factor is defined as the ratio of
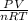 here P is pressure V is volume R is constant and n is number of moles and T is temperature the ratio of
here P is pressure V is volume R is constant and n is number of moles and T is temperature the ratio of
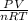 is 1 for the ideal gas. The compressibility factor is denoted by Z , its value may be greater or less than one but for ideal gas it is always 1
is 1 for the ideal gas. The compressibility factor is denoted by Z , its value may be greater or less than one but for ideal gas it is always 1