Answer:
a. Isotopes
Step-by-step explanation:
If we have same atom but with different number of neutrons then in that case the number of neutrons will decide the mass number of the atom
Mass number = Number of protons + Number of Neutrons
so here we can say that the mass number of the two same atoms would be different while the atomic number would be same
for an example we have
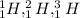
so in this example all are hydrogen atom but all have different number of neutrons
all such group of atoms is known as isotopes