Answer:
8000 L/ mol cm is the value of the molar absorptivity coefficient.
Step-by-step explanation:
The slope of the absorbance- concentration graph is equal to the product of path length and molar absorptivity coefficient.
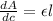
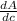 = Rate of change of absorbance with respect to concentration
= Rate of change of absorbance with respect to concentration
 = molar absorptivity coefficient
= molar absorptivity coefficient
l = Path-length
Given the slope of graph of absorbance vs procaine hydrochloride concentration.
y = 240000 x (linear equation)
Path length of cuvette = 3 cm
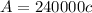
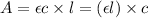
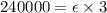
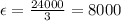
The units of
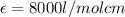
8000 L/ mol cm is the value of the molar absorptivity coefficient.