Answer:
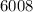 cal
cal
Step-by-step explanation:
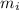 = mass of ice = 100 g = 0.1 kg
= mass of ice = 100 g = 0.1 kg
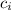 = specific heat of ice = 0.5 cal/(kg°C)
= specific heat of ice = 0.5 cal/(kg°C)
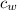 = specific heat of water = 1 cal/(kg°C)
= specific heat of water = 1 cal/(kg°C)
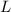 = Latent heat of fusion of ice = 80 J/g
= Latent heat of fusion of ice = 80 J/g
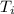 = initial temperature of ice = - 20 °C
= initial temperature of ice = - 20 °C
 = final temperature of ice = 50 °C
= final temperature of ice = 50 °C
Q = Heat gained
Heat gained is given as
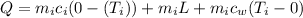
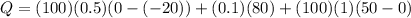
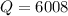 cal
cal