Answer:
Step-by-step explanation:
A)
W = work done by the freezer = 800 J
Q = heat removed from the freezer = 1735 J
Q' = Heat expelled into the room
Coefficient of performance is given as
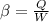
inserting the values
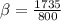
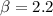
B)
Heat expelled is given as
Q' = W + Q
Q' = 800 + 1735
Q' = 2535 J