Answer:
Frequency = 3.5294×10¹⁴ s⁻¹
Wavenumber = 1.1765×10⁶ m⁻¹
Energy = 2.3365x 10⁻¹⁹ J , 1.4579 eV , 2.3365x 10⁻¹² erg
Step-by-step explanation:
Given the wavelength = 850 nm
1 nm = 10⁻⁹ m
So, wavelength is 850×10⁻⁹ m
The relation between frequency and wavelength is shown below as:
c = frequency × Wavelength
Where, c is the speed of light having value = 3×10⁸ m/s
So, Frequency is:
Frequency = c / Wavelength
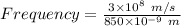
Frequency = 3.5294×10¹⁴ s⁻¹
Wavenumber is the reciprocal of wavelength.
So,
Wavenumber = 1 / Wavelength = 1 / 850×10⁻⁹ m
Wavenumber = 1.1765×10⁶ m⁻¹
Also,
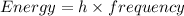
where, h is Plank's constant having value as 6.62x 10⁻³⁴ J.s
So,
Energy = 6.62x 10⁻³⁴ J.s × 3.5294×10¹⁴ s⁻¹
Energy = 2.3365x 10⁻¹⁹ J
Also,
1 J = 6.24×10¹⁸ eV
So,
Energy = 2.3365x 10⁻¹⁹ × 6.24×10¹⁸ eV
Energy = 1.4579 eV
Also,
1 J = 10⁷ erg
So,
Energy = 2.3365x 10⁻¹⁹ × 10⁷ erg
Energy = 2.3365x 10⁻¹² erg