Step-by-step explanation:
Moles of sucrose =
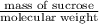
=
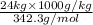
=
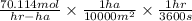
=
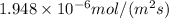
Energy required for photosynthesis = 5640 kJ/mol
Energy needed to produce 24 kg sucrose
= Energy required for photosynthesis x Moles of sucrose
=
![(5640 kJ/mol) * [1.948 x 10^(-6) mol/(m^(2)s)]](https://img.qammunity.org/2020/formulas/chemistry/college/q1y4yxmqxjh3qj7xj5bnpeypufcouzdols.png)
=
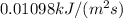
Energy supplied from sun =
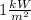
= 1
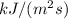
Efficiency for photosynthesis =
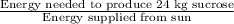
=
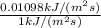
=
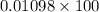
= 1.098%
Thus, we can conclude that efficiency of photosynthesis for a plant that is being considered as a source of biofuels is 1.098 %.