Answer:
Condensed steam, M = 0.091 g
The remaining steam = 199.9 g
Given:
mass of lead, m = 50 g
temperature, T = -10

mass of steam, m' = 200 g
temperature, T' = 105

Solution:
Now,
latent heat of vaporization of steam, L = 2260 J/g
specific heat of lead,
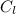 = 0.128 J/g-K
= 0.128 J/g-K
specific heat of steam,
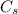 = 1.996 J/g-K
= 1.996 J/g-K
Now, absorbed heat of lead to reach
![0^{\circ]](https://img.qammunity.org/2020/formulas/physics/college/t2xgdx0raiv4pdwzprfw7ceyj8arxm2j6f.png) :
:
q = m
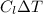
q =
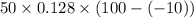
q =
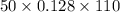
q = 704 J
Now, to reach 100
 the amount of heat released by steam:
the amount of heat released by steam:
q' = m'
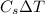
q' =
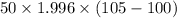
q' = 499 J
Now,
Let the condensed part of the steam have mass M, then:
ML = q - q'
M =
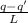
M =
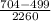
M = 0.091 g
The remaining steam = 200 - 0.091 = 199.9 g