Step-by-step explanation:
The given data is as follows.
Pressure of steam at inlet of turbine,
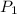 = 20 bar
= 20 bar
Temperature at inlet of turbine, T =
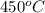
Pressure at outlet of turbine,
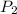 = 10 bar
= 10 bar
Mass flow rate of steam, m = 200 kg/min
Work produced by the turbine,
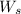 = 1500 kW
= 1500 kW
Steam is heated at constant pressure to its initial temperature, i.e., temperature at outlet of heat exchanger,
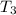 =
=
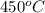 .
.
(1) For an adiabatic turbine, the energy balance is as follows.
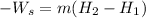
where
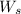 = work done by the turbine
= work done by the turbine
m = mass flow rate of steam
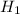 and
and
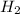 are the specific enthalpy of steam at inlet and outlet conditions of turbine.
are the specific enthalpy of steam at inlet and outlet conditions of turbine.
Obtain the specific enthalpy of steam from Properties of Superheated Steam table
At 20 bar and
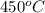 ,
,
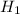 = 3358 kJ/kg
= 3358 kJ/kg
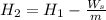
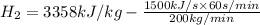
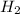 = 2908 kJ/kg
= 2908 kJ/kg
For P = 10 bar,
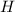 =2875 kJ/kg for T=
=2875 kJ/kg for T=
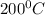 and H = 2975 kJ/kg for
and H = 2975 kJ/kg for
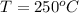 . Interpolate the values.
. Interpolate the values.
The temperature corresponding to P = 10 bar and
 = 2908 kJ/kg is T =
= 2908 kJ/kg is T =
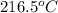
Therefore, the outlet temperature is
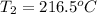 .
.
(2) Energy balance on the heater is as follows.
Q =
 =
=
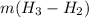
where, Q = heat input required by the steam
 = specific enthalpy change
= specific enthalpy change
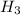 = specific enthalpy of steam at the outlet conditions of heat exchanger
= specific enthalpy of steam at the outlet conditions of heat exchanger
At P = 10 bar and
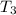 =
=
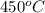 ,
,
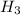 = 3371 kJ/kg.
= 3371 kJ/kg.
Q =
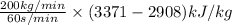
[/tex]
Q = 1543.33 kJ/s
or, Q = 1543.33 kW
Therefore, the heat input required is Q = 1543.33 kW.