Step-by-step explanation:
(a) As we know that relation between normality and volume of two solutions is as follows.
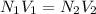
As the given data is as follows.
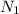 = ?,
= ?,
 = 0.050 N
= 0.050 N
 = 20 ml,
= 20 ml,
 = 16.25 ml
= 16.25 ml
Therefore, putting the given values into the above formula as follows.
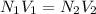
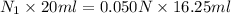
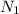 = 0.0406 N
= 0.0406 N
Hence, normality of solution A is 0.0406 N.
(b) As gram equivalent weight of KBr is 119 g/mol.
Formula to calculate normality is as follows.
Normality =
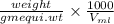
As volume is 200 ml, normality is 0.0406 N, and gram equi. wt is 119 g/mol. Therefore, putting these values into the above formula as follows.
Normality =
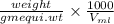
0.0406 N =
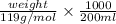
weight = 0.9668 g
As 0.9668 g of KBr is present in 1.1250 g sample. Hence, weight percentage will be calculated as follows.
Weight % of KBr =
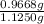
= 85.94%
Thus, we can conclude that C% w/w of KBr in the original sample is 85.94%.