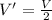Answer:
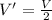
So volume is halved
Step-by-step explanation:
As we know that heat energy given to the gas is equal to the work done
so as per first law of thermodynamics we know that

now we know that

so we have
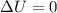
so we have temperature of the gas is constant
so it is an isothermal process
so here we can say
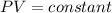
so here we can say
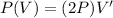
so we have