Step-by-step explanation:
As it is given that solubility of water in diethyl ether is 1.468 %. This means that in 100 ml saturated solution water present is 1.468 ml.
Hence, amount of diethyl ether present will be calculated as follows.
(100ml - 1.468 ml)
= 98.532 ml
So, it means that 98.532 ml of diethyl ether can dissolve 1.468 ml of water.
Hence, 23 ml of diethyl ether can dissolve the amount of water will be calculated as follows.
Amount of water =
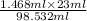
= 0.3427 ml
Now, when magnesium dissolves in water then the reaction will be as follows.
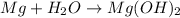
Molar mass of Mg = 24.305 g
Molar mass of
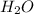 = 18 g
= 18 g
Therefore, amount of magnesium present in 0.3427 ml of water is calculated as follows.
Amount of Mg =
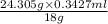
= 0.462 g