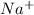Answer and Explanation:
The elements which have 1 2 or 3 valance electron in their valence cell those elements loses their electron to maintain their octet and for becoming stable and, if they loses electron positive charge will create and these ions are called cation
Example : In ionic compound
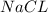 Na has 1 valance electron which it is losses and form
Na has 1 valance electron which it is losses and form