Answer:
a) 283 K
b) 136825 Pa
c) 15.992
d) 1878.6 m/s
Step-by-step explanation:
a)
T = absolute temperature = 10 °C = 10 + 273 = 283 K
b)
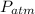 = Atmospheric pressure = 101325 Pa
= Atmospheric pressure = 101325 Pa
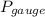 = gauge pressure = 35500 Pa
= gauge pressure = 35500 Pa
Absolute pressure is given as
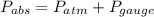
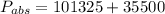
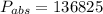 Pa
Pa
c)
 = number of moles of Hydrogen
= number of moles of Hydrogen
 = Volume of hydrogen = 0.275 m³
= Volume of hydrogen = 0.275 m³
Using the equation
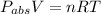
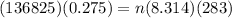
 = 15.992
= 15.992
d)
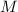 = Molar mass of hydrogen = 2 g mol⁻¹ = 0.002 kg mol⁻¹
= Molar mass of hydrogen = 2 g mol⁻¹ = 0.002 kg mol⁻¹
 = root mean square speed
= root mean square speed
Root mean square speed is given as
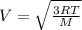
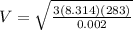
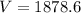 m/s
m/s