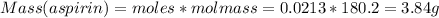Answer:
Theoretical Yield of Aspirin = 3.84 g
Step-by-step explanation:
The reaction between salicylic acid (C7H6O3) and acetic anhydride (C4H6O3) to form aspirin(C9H8O4) and acetic acid (HC2H3O2) is:
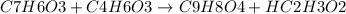
Mass of salicylic acid present = 2.94 g
Molar Mass of salicylic acid = 138.1 g/mol
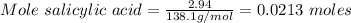
Since acetic anhydride is in excess, salicylic acid will be the limiting reagent and will determine the amount of product formed.
Based on the reaction stoichiometry:
1 mole of salicylic acid yields 1 mole of aspirin
Therefore, Moles of aspirin = 0.0213
Molar mass of aspirin = 180.2 g/mol