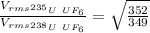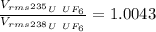Answer:
1.0043
Step-by-step explanation:
The formula for root mean square velocity is:
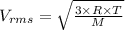
Where,
R is the universal gas constant
T is the temperature
M is the molecular weight
Since, seen from the formula, root mean square velocity is inversely proportional to the square root of the molecular mass.
Thus, for two gases like
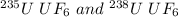 .The expression is:
.The expression is:
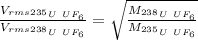
The molecular mass of
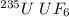 is 349.0 g/mol
is 349.0 g/mol
The molecular mass of
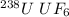 is 352.0 g/mol
is 352.0 g/mol