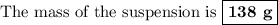Answer:
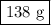
Step-by-step explanation:
Ca(s) + 2H₂O(ℓ) ⟶ Ca(OH)₂(s) + H₂(g)
Mass of reactants = mass of products
You have 140 g of reactants, so you should get 140 g of products.
The calcium hydroxide and the left-over water will remain in the beaker, but the hydrogen will escape.
According to the equation, 1 mol (40 g) of Ca produces 1 mol (2.0 g) of hydrogen.
Mass of suspension + mass of hydrogen = 140 g
Less mass of hydrogen = 2.0 g
Mass of suspension = 138 g