Answer: Option (c) is the correct answer.
Step-by-step explanation:
Isotopes are the species that contain same number of protons but different number of neutrons.
For example, isotopes of carbon are
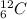 and
and
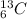 .
.
So, both these atoms contain six protons but different number of neutrons.
As sum of total number of protons and neutrons present within an element is known as atomic mass.
Hence, number of neutrons can be calculated as follows.
Atomic mass = atomic number + no. of neutrons
No. of neutrons = atomic mass - atomic number
Thus, we can conclude that scientists determine the number of neutrons in an isotope of an atom as they subtract the number of protons from the mass number.