Answer:
Part 1: A and B; Part 2: A and D
Step-by-step explanation:
Part 1. Evidence of reaction
The evolution of heat and/or bubbles of gas are evidence that a reaction has occurred.
C is wrong. If adding yellow Solution A to yellow Solution B gives a yellow mixture, that is not evidence of reaction. You need a colour change to provide evidence.
Part 2. Identifying reactants and products
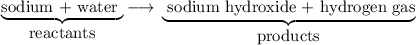
Reactants are on the left side of the reaction arrow, and products are on the right.
Thus, water is a reactant, and sodium hydroxide is a product.
B is wrong. Sodium is a reactant.