Answer:
True
Step-by-step explanation:
Pauli exclusion principle states that in an atom, no two electrons have same set of the quantum numbers. The electrons in an atom have a unique set of quantum numbers ,
Even the electron is in same shell, same subshell and same orbital, the spin quantum number will be different for the two of them.
For example : 2 electrons in
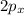
n = 2 , l = 1 , m = 1 but s is different for both of them . (+1/2 or - 1/2)