Step-by-step explanation:
It is given that,
Voltage,
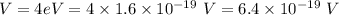
De broglie wavelength in terms of voltage is given by :
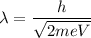
m and e are the mass and charge on electron. So,
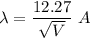
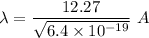
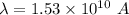
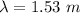
So, the De broglie wavelength of an electron is 1.53 meters. Hence, this is the required solution.