Answer
When an electron makes transition from a state of higher energy to a state of lower energy it does so by emitting energy in form of radiation in the visible spectrum of light.
Since the basic postulates of the atomic theory is that the energy that the electron possess in it's orbit's takes only discrete values and cannot take any random value thus when an electron makes a transition from a state of higher energy to state of lower energy it will emit radiation with energy equal to difference between the energy levels of the 2 orbit's thus we only observe discrete lines.
Mathematically when an electron makes a transition between states the wavelength of light it releases is given by
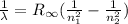
where
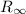 is Rydberg constant
is Rydberg constant
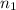 is upper energy level
is upper energy level
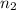 is lower energy level
is lower energy level
thus we can see that only discrete wavelength's are released and not continuous wavelength's of light.