Step-by-step explanation:
The given data is as follows.
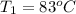 = (83 + 273) K = 356 K
= (83 + 273) K = 356 K
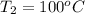 = (100 + 273) K = 373 K
= (100 + 273) K = 373 K
m = 3.9 kg/s
Relation between heat energy and specific heat is as follows.
Q =
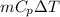
Putting the given values into the above formula as follows.
Q =
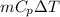
=
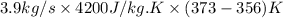
= 278460 J/s
As it is given that enthalpy of evaporation of water is 2760000 J/kg.
Hence, energy left is as follows.
(2760000 - 278460) J/s
= 2481540 J/s
So, enthalpy of vapor energy at
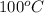 is as follows.
is as follows.
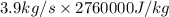
= 10764000 J/s
Hence, energy left for steam is as follows.
(10764000 J/s - 2481540 J/s)
= 8282460 J/s
Now, steam is formed at
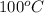 or (100 + 273)K = 373 K. Therefore, final temperature will be calculated as follows.
or (100 + 273)K = 373 K. Therefore, final temperature will be calculated as follows.
Q =
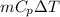
8282460 J/s =
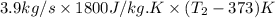
 = 1552.83 K
= 1552.83 K
Thus, we can conclude that the temperature of steam leaving the pipe is 1552.83 K.