Answer:
Ksp = 3.4 × 10⁻⁷
Step-by-step explanation:
First, we will express the solubility in molarity (molar mass = 183.68 g/mol).
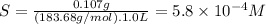
Let's consider the solution of SrSO₄.
SrSO₄(s) ⇄ Sr⁺²(aq) + SO₄²⁻(aq)
To relate the solubility (S) to the solubility product (Ksp) we will use an ICE chart. We recognize 3 stages (Initial, Change, Equilibrium) and complete each row with the concentration or change in the concentration.
SrSO₄(s) ⇄ Sr⁺²(aq) + SO₄²⁻(aq)
I 0 0
C +S +S
E S S
Ksp = [Sr⁺²].[SO₄²⁻] = S² = (5. 8 × 10⁻⁴)² = 3.4 × 10⁻⁷