Answer:
Q = -68.859 kJ
Step-by-step explanation:
given details
mass
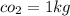
initial pressure P_1 = 104 kPa
Temperature T_1 = 25 Degree C = 25+ 273 K = 298 K
final pressure P_2 = 1068 kPa
Temperature T_2 = 311 Degree C = 311+ 273 K = 584 K
we know that
molecular mass of
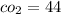
R = 8.314/44 = 0.189 kJ/kg K
c_v = 0.657 kJ/kgK
from ideal gas equation
PV =mRT
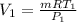
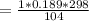
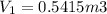
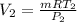
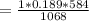

WORK DONE
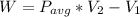
w = 586*(0.1033 -0.514)
W =256.76 kJ
INTERNAL ENERGY IS
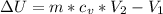
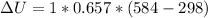
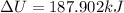
HEAT TRANSFER
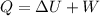
= 187.902 +(-256.46)
Q = -68.859 kJ