Answer:
Correct option is (a).
Step-by-step explanation:
Solubility equilibrium of ZnS is represented as-
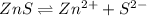
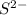 is a strong conjugate base of weak acid
is a strong conjugate base of weak acid
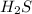 .
.
Therefore addition of HCl in ZnS results formation of
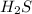 . Thus
. Thus
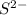 is removed as
is removed as
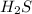 .
.
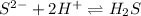
So, ZnS tends to be more soluble with addition of dilute HCl to keep constant solubility product.