Step-by-step explanation:
The given data is as follows.
Flow of mass rate of hydrocarbon, m = 0.126 kg/s
Heat of condensation, (
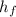 ) = 335 kJ/kg
) = 335 kJ/kg
For water
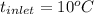 , and
, and
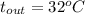
Formula for transfer of heat is as follows.
Q =
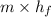
=
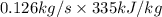
= 41.875 kJ/s
Also, it is known that Q =
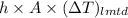
As it is given that for condensing T =
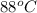 .
.
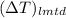 =
=
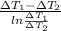
=
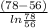
=
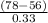
=
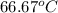
As it is given that inside and outside diameters of the tube are as follows.
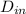 = 0.0127, and
= 0.0127, and
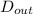 = 0.0152 m
= 0.0152 m
Formula for A =
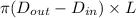 ......... (1)
......... (1)
Hence, putting value of equation (1) in the following formula.
Q =
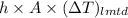
41.875 kJ/s =
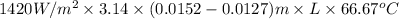
L = \frac{41.875 \times 1000 J/s}{743.17 W^{o}C}
= 56.34 h
Thus, we can conclude that length of copper tubing will be required to accomplish the desired heat transfer 56.34 h.