Answer:
D = 18000 kg/m3
V = 2.5*10{-7}m3
Step-by-step explanation:
From the Archimedes principle,
Weight of fluid displaced = W_{air} - W_{water}
W_{air} = 4.5 gm
W_{water} = 4.25 gm
![W = [4.5 - 4.25]*9.81*10^(-3)](https://img.qammunity.org/2020/formulas/physics/college/xojlz72bcs20p5gj29igp3mrn263iqub2z.png)
W = 2.4525*10{-3} N
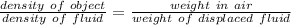
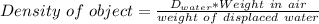
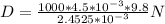
D = 18000 kg/m3
b) object Volume can be obtained as ,
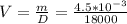
V = 2.5*10{-7}m3