Answer: a) the reaction has not occurred
b) the reaction is in equilibrium
c) the reaction is very much product favored
Explanation:
Equilibrium constant is the ratio of the concentration of products to the concentration of reactants each term raised to its stochiometric coefficients.
For a general chemical reaction:
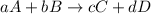
The expression for
 is written as:
is written as:
![K_(eq)=([C]^c[D]^d)/([A]^a[B]^b)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/i3xubhuf1a7gm3aqx745lvdzgt15yry9zu.png)
There are 3 conditions:
When
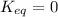 ; the reaction has not occurred, the concentration of products is zero
; the reaction has not occurred, the concentration of products is zero
When
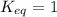 ; the reaction is in equilibrium, the concentration of products is equal to that of reactants
; the reaction is in equilibrium, the concentration of products is equal to that of reactants
When
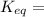 ; the reaction is very much product favored, the concentration of products is much higher than that of reactants
; the reaction is very much product favored, the concentration of products is much higher than that of reactants
From the above expression, the equilirbium constant is directly dependent on product concentration. Thus, more is the concentration of product, more will be the equilibrium constant.
The highest values of
 will favor the product more.
will favor the product more.