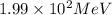Answer:
For a: The isotopic symbol of unknown element is
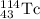
For b: The amount of mass lost during this process is 0.2137730 u.
For c: The energy released in the given nuclear reaction is
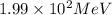
Step-by-step explanation:
In a nuclear reaction, the total mass and total atomic number remains the same.
For the given fission reaction:
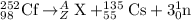
Total mass on reactant side = total mass on product side
252 = A + 135 + 3
A = 114
Total atomic number on reactant side = total atomic number on product side
98 = Z + 55 + 0
Z = 43
Hence, the isotopic symbol of unknown element is
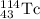
We are given:
Mass of
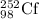 = 252.081626 u
= 252.081626 u
Mass of
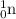 = 1.008665 u
= 1.008665 u
Mass of
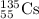 = 134.905978 u
= 134.905978 u
Mass of
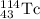 = 113.935880 u
= 113.935880 u
To calculate the mass defect, we use the equation:
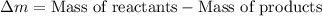
Putting values in above equation, we get:
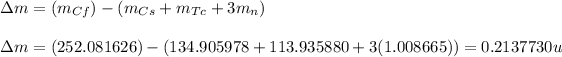
Hence, the amount of mass lost during this process is 0.2137730 u.
To calculate the energy released, we use the equation:
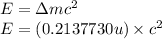
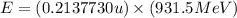 (Conversion factor:
(Conversion factor:
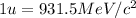 )
)

Hence, the energy released in the given nuclear reaction is