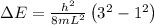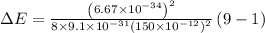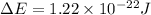Answer:
\Delta E=1.22\times 10^{-22}J
Step-by-step explanation:
The energy of electron in any state is given by
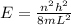 here h is planck's constant n is state of electron L is the infinte potential well m is the mass of electron
here h is planck's constant n is state of electron L is the infinte potential well m is the mass of electron
We know that
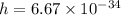
Potential well dimension =
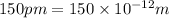
Mass of electron
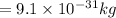
So energy required to electron to jump from ground state to 3rd state