Step-by-step explanation:
(a) It is given that heat transferred is -546 kJ (as heat is releasing) and temperature is 110 degree celsius.
Hence, the temperature in kelvin will be as follows.
(110 + 273) K
= 383 K
As it is known that relation between entropy change and heat is as follows.
S =
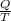
Putting the given values in the above equation as follows.
S =
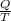
=
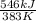
= -1.4255 kJ/K
As temperature changes from
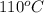 to
to
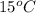 . So, (15 + 273) K = 288 K. Hence, change in entropy will be calculated as follows.
. So, (15 + 273) K = 288 K. Hence, change in entropy will be calculated as follows.
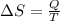
=
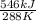
= 1.895 kJ/K
Therefore, entropy change of water will be 1.895 kJ/K.
(b) As total entropy generated will be the sum of both the entropies as follows.
Total entropy = (-1.4255 kJ/K + 1.895 kJ/K)
= 0.4695 kJ/K
Thus, total entropy during this heat transfer process is 0.4695 kJ/K.