Answer: The Fermi velocity of lead is 64.4 km/s.
Step-by-step explanation:
To calculate the Fermi velocity, we use the equation:
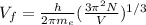
where,
h = Planck's constant =
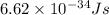
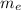 = mass of electron =
= mass of electron =
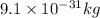
N = Number of atoms present in per volume of atom multiplied by number of electrons present in given atom =
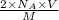
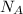 = Avogadro's number =
= Avogadro's number =
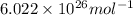 (When the mass is in kilograms)
(When the mass is in kilograms)
V = Volume =
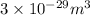
M = molecular weight of lead = 207.2 g/mol
Putting values in above equation, we get:
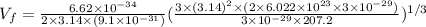
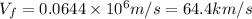 (Conversion factor: 1 km = 1000 m)
(Conversion factor: 1 km = 1000 m)
Hence, the Fermi velocity of lead is 64.4 km/s