Answer:
CHANGE IN VOLUME -0.013 m3
Step-by-step explanation:
given data:
we know that pressure remain constant for weighted piston cylinder
P1 =P2
Gas constant R = 259.81 j/Kg- K = 0.2598 kJ/kg K
mass, m1 =m2 = 0.01 kg
P1 = 20kPa
T1 = 100 degree C = 373 K
T2 = 0 degree C= 273 K
FROM IDEAL EQUATION
P1V1 =m RT1
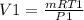

V1 = 0.0484 m3
P2V2 =m RT2
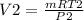
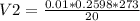
V2 = 0.0.3546 m3
CHANGE IN VOLUME OS GIVEN
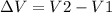
= 0.0354 - 0.0484
= -0.013 m3