Step-by-step explanation:
As it is given that process is entropic which means that it is reversible adiabatic in nature.
Also, the given data is as follows.
 =
=
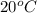 = (20 + 273) K = 293 K
= (20 + 273) K = 293 K
Gage pressure = 150 kPa, Atmospheric pressure = 101.3 kPa
Hence, pressure (
 ) will be (150 kPa + 101.3 kPa) = 251.3 kPa
) will be (150 kPa + 101.3 kPa) = 251.3 kPa
and, pressure (
 ) will be (325 kPa + 101.3 kPa) = 426.3 kPa
) will be (325 kPa + 101.3 kPa) = 426.3 kPa
Also, specific heat ratio (
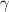 )is given as 1.40.
)is given as 1.40.
Hence, relation between T and P is as follows.
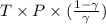 = constant
= constant
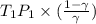 =
=

 =
=

=

= 340.758 K
Hence, convert this temperature into degree celsius as follows.

=
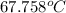
Thus, we can conclude that the temperature is
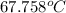 .
.